Family Dog Project
Research groups
History of the Family Dog Project

The Family Dog Project is an umbrella term for dog-related research groups studying domestic dogs' behaviour, brain, cognition and evolution, based at the Department of Ethology, Eötvös Loránd University or the ELKH TTK KPI in Budapest. The very first group was founded in the 1990s by Vilmos Csányi and later led by Ádám Miklósi, ethologists and evolutionary biologists, and has since become one of the world's leading research groups in the field of dog behaviour and cognition.
The Family Dog Project conducts both basic and applied research, with the aim of understanding how dogs interact with humans and how they evolved to become one of our closest animal companions. Their research explores topics such as social behaviour, ageing, communication, problem-solving, memory and emotions in dogs, occasionally compared to wolves, cats and mini-pigs. They also investigate the relationship between dogs and humans, including how dogs perceive and respond to human communication and how humans can better communicate with their dogs.
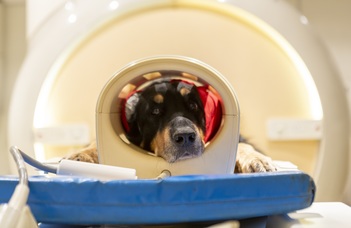
The Family Dog Project is known for using innovative research methods such as eye-tracking and neuroimaging to study dog behaviour and cognition. Their research has shed new light on the remarkable abilities of dogs and has practical implications for improving dog welfare and the human-dog bond. Please check the Research Groups in the Menu.

Ádám Miklósi, Vilmos Csányi and Janka
Research topics
Last updated in 2015
Comparative psychology
- Topál, J., Miklósi, Á., Gácsi, M., Dóka, A., Pongrácz, P., Kubinyi, E., Virányi Zs., Csányi, V. 2009. The dog as a model for understanding human social behavior. Advances in the Study of Animal Behaviour, 39: 71-116.
- Miklósi, Á., Topál, J. 2013. What does it take to become ‘best friends’? Evolutionary changes in canine social competence. Trends in Cognitive Sciences, 17: 287-294.
Attachment
- Topál, J., Miklósi, Á., Csányi, V. 1998. Attachment behaviour in dogs: a new application of Ainsworth’s (1969) Strange Situation Test. Journal of Comparative Psychology, 112: 219-229.
- Gácsi, M., Topál, J., Miklósi, Á., Dóka, A., Csányi, V. 2001. Attachment behaviour of adult dogs (Canis familiaris) living at rescue centres: Forming new bonds. Journal of Comparative Psychology, 115: 423-431.
- Naderi, Sz., Miklósi, Á., Dóka, A., Csányi, V. 2002. Does dog-human attachment affect their inter-specific cooperation? Acta Biologica Hungarica, 53: 537-550.
- Topál, J., Gácsi, M., Miklósi, Á., Virányi, Zs., Kubinyi, E., Csányi, V. 2005. Attachment to humans: a comparative study on hand-reared wolves and differently socialized dog puppies. Animal Behaviour, 70: 1367-1375.
- Kovács, Sz., Gácsi, M., Vincze, D., Korondi, P., Miklósi, Á. 2011. A novel, ethologically inspired HRI model implementation: Simulating dog-human attachment. In: 2nd International Conference on Cognitive Infocommunications: CogInfoCom, Budapest, Hungary, IEEE Computer SocietyPress, pp. 1-4. (ISBN:9781457718069)
- Gácsi, M., Maros, K., Sernkvist, S., Faragó, T., Miklósi, Á. 2013. Human analogue safe haven effect of the owner: behavioural and heart rate response to stressful social stimuli in dogs. PLoS ONE, 8(3): e58475.
- Kis, A., Klausz, B., Persa, E., Miklósi, Á., Gácsi, M. 2014. Timing and presence of an attachment person affect sensitivity of aggression tests in shelter dogs. Veterinary Record, 174: 196.
Pointing gestures
- Soproni, K., Miklósi, Á., Topál, J., Csányi, V. 2002. Dogs’ responsiveness to human pointing gestures. Journal of Comparative Psychology, 116: 27-34.
- Miklósi, Á., Soproni, K. 2006. A comparative analysis of animals’ understanding of the human pointing gesture. Animal Cognition, 9: 81-93.
- Lakatos, G., Dóka, A., Miklósi, Á. 2007. The role of visual cues in the comprehension of the human pointing signals in dogs. International Journal of Comparative Psychology, 20: 341-350.
- Virányi, Zs., Gácsi, M., Kubinyi, E., Topál, J., Belényi, B., Ujfalussy, D., Miklósi, Á. 2008. Comprehension of human pointing gestures in young human-reared wolves (Canis lupus) and dogs (Canis familiaris). Animal Cognition, 11: 373-387.
- Maros, K., Gácsi, M., Miklósi, Á. 2008. Comprehension of human pointing gestures in horses (Equus caballus). Animal Cognition, 11: 457-66.
- Lakatos, G., Soproni, K., Dóka, A., Miklósi, Á. 2009. A comparative approach to dogs’ (Canis familiaris) and human infants’ comprehension of various forms of pointing gestures. Animal Cognition, 12: 621-631.
- Gácsi, M., Kara, E., Belényi, B., Topál, J., Miklósi, Á. 2009. The effect of development and individual differences in pointing comprehension of dogs. Animal Cognition, 12: 471-479.
- Gácsi, M., Győri, B., Virányi, Zs., Kubinyi, E., Range, F., Belényi, B., Miklósi, Á. 2009. Explaining dog wolf differences in utilizing human pointing gestures: Selection for synergistic shifts in the development of some social skills. PLoS ONE 4(8): e6584.
- Gácsi M, McGreevy P, Kara E, Miklósi Á. 2009. Effects of selection for cooperation and attention in dogs. Behavior and Brain Functions. 5:31.
- Lakatos, G., Gácsi, M., Topál, J., Miklósi, Á. 2012. Comprehension and utilisation of pointing gestures and gazing in dog–human communication in relatively complex situations. Animal Cognition, 15: 201-213.
- Pongrácz, P., Gácsi, M., Hegedüs, D., Péter, A., Miklósi, Á. 2013. Test sensitivity is important for detecting variability in pointing comprehension in canines. Animal Cognition, 16: 721-735.
- Pfandler, E., Lakatos, G., Miklósi, Á. 2013. Eighteen-month-old human infants show intensive development in comprehension of different types of pointing gestures. Animal Cognition, 16: 711-719.
- Hegedüs, D., Bálint, A., Miklósi, Á., Pongrácz, P. 2013. Owners fail to influence the choices of dogs in a two-choice, visual pointing task. Behaviour, 150: 427–443.
Guide dogs for the blinds
- Naderi, Sz., Miklósi, Á., Dóka, A., Csányi, V. 2001. Co-operative interactions between blind persons and their dogs. Applied Animal Behaviour Science, 74: 59-80.
- Naderi, Sz., Miklósi, Á., Dóka, A., Csányi, V. 2002. Does dog-human attachment affect their inter-specific cooperation? Acta Biologica Hungarica, 53: 537-550.
Social learning (from social anticipation to imitation)
- Pongrácz, P., Miklósi, Á., Kubinyi, E., Gurobi, K., Topál, J., Csányi, V. 2001. Social learning in dogs: The effect of a human demonstrator on the performance of dogs (Canis familiaris) in a detour task. Animal Behaviour, 62: 1109-1117.
- Kubinyi, E., Miklósi, Á., Topál, J., Csányi, V. 2003. Social mimetic behaviour and social anticipation in dogs: preliminary results. Animal Cognition, 6: 57-63.
- Kubinyi, E., Topál, J., Miklósi, Á., Csányi, V. 2003. Dogs (Canis familiaris) learn from their owners via observation in a manipulation task. Journal of Comparative Psychology, 117: 156-165.
- Pongrácz, P., Miklósi, Á., Kubinyi, E., Topál, J., Csányi, V. 2003. Interaction between individual experience and social learning in dogs. Animal Behaviour, 65: 595-603.
- Pongrácz, P., Miklósi, Á., Timár-Geng K., Csányi V. 2004. Verbal attention getting as a key factors in social learning between dog (Canis familiaris) and human. Journal of Comparative Psychology, 118: 375-383.
- Pongrácz, P., Vida, V., Bánhegyi, P., Miklósi, Á. 2008. How does dominance rank status affect individual and social learning performance in the dog (Canis familiaris)? Animal Cognition, 11: 75-82.
- Kubinyi, E., Pongrácz, P., Miklósi, Á. 2009. Dog as a model for studying con- and heterospecific social learning. Journal of Veterinary Behavior, 4: 31-41.
- Topál, J., Byrne, R.W., Miklósi, Á., Csányi, V. 2006. Reproducing human actions and action sequences: „Do as I Do!” in a dog. Animal Cognition, 9: 355-367.
- Huber, L., Range, F., Voelkl, B., Szucsich, A., Virányi, Zs., Miklósi, Á. 2009. The evolution of imitation: what do the capacities of non-human animals tell us about the mechanisms of imitation? Phil. Trans. R. Soc. B., 364: 2299-2309.
- Fugazza, C., Miklósi, Á. 2014. Should old dog trainers learn new tricks? The efficiency of the Do as I do method and shaping/clicker training method to train dogs. Applied Animal Behaviour Science, 153: 53-61.
- Fugazza, C., Miklósi, Á. 2014. Deferred imitation and declarative memory in domestic dogs.Animal Cognition, 17: 237-247.
Dog-wolf comparisons
- Miklósi, Á., Kubinyi E., Topál, J., Gácsi, M., Virányi, Zs., Csányi, V. 2003. A simple reason for a big difference: wolves do not look back at humans but dogs do. Current Biology, 13: 763-766.
- Topál, J., Gácsi, M., Miklósi, Á., Virányi, Zs., Kubinyi, E., Csányi, V. 2005. Attachment to humans: a comparative study on hand-reared wolves and differently socialized dog puppies. Animal Behaviour, 70: 1367-1375.
- Gácsi, M., Győri, B., Miklósi, Á., Virányi, Zs., Kubinyi, E., Topál, J., Csányi, V. 2005. Species-specific differences and similarities in the behavior of hand-raised dog and wolf pups in social situations with humans. Developmental Psychobiology, 47: 111-122.
- Kubinyi, E., Virányi, Zs., Miklósi, Á. 2007. Comparative social cognition: From wolf and dog to humans. Comparative Cognition & Behavior Reviews, 2: 26-46.
- Virányi, Zs., Gácsi, M., Kubinyi, E., Topál, J., Belényi, B., Ujfalussy, D., Miklósi, Á. 2008. Comprehension of human pointing gestures in young human-reared wolves (Canis lupus) and dogs (Canis familiaris). Animal Cognition, 11: 373-387.
- Gácsi, M., Győri, B., Virányi, Zs., Kubinyi, E., Range, F., Belényi, B., Miklósi, Á. 2009. Explaining dog wolf differences in utilizing human pointing gestures: Selection for synergistic shifts in the development of some social skills. PLoS ONE 4(8): e6584.
- Topál, J., Gergely, Gy., Erdőhegyi, Á., Csibra, G., Miklósi, Á. 2009. Differential sensitivity to human communication in dogs, wolves, and human infants. Science, 325: 1269-1272.
- Topál, J., Miklósi, Á., Sümegi, Zs., Kis, A. 2010. Response to comments on „Differential sensitivity to human communication in dogs, wolves and human infants.” Science, 329: 142d.
- Gácsi, M., Vas, J., Topál, J., Miklósi, Á. 2013. Wolves do not join the dance: Sophisticated aggression control by adjusting to human social signals in dogs. Applied Animal Behaviour Science, 145: 109-122.
Rule following
- Watson, J.S., Gergely, G., Topál, J., Gácsi, M., Sárközi, Zs., Csányi, V. 2001. Distinguishing logic from association in the solution of an invisible displacement task by children and dogs: Using negation of disjunction. Journal of Comparative Psychology, 115: 219-226.
- Kubinyi, E., Miklósi, Á., Topál, J., Csányi, V. 2003. Social mimetic behaviour and social anticipation in dogs: preliminary results. Animal Cognition, 6: 57-63.
- Topál, J., Kubinyi, E., Gácsi, M., Miklósi, Á. 2005. Obeying social rules: A comparative study on dogs and humans. Journal of Cultural and Evolutionary Psychology, 3: 213-239.
- Topál, J., Gergely, Gy., Miklósi, Á., Erdőhegyi, Á., Csibra, G. 2008. Infants’ perseverative search errors are induced by pragmatic misinterpretation. Science, 321: 1831-1834.
- Topál, J., Tóth, M., Gergely, Gy., Csibra, G. 2009. Response to comment on „Infants perseverative search errors are induced by pragmatic misinterpretation.” Science, 325: 1624.
- Topál, J., Gergely, Gy., Erdőhegyi, Á., Csibra, G., Miklósi, Á. 2009. Differential sensitivity to human communication in dogs, wolves, and human infants. Science, 325: 1269-1272.
- Topál, J., Miklósi, Á., Sümegi, Zs., Kis, A. 2010. Response to comments on „Differential sensitivity to human communication in dogs, wolves and human infants.” Science, 329: 142d.
- Kis, A., Topál, J., Gácsi, M., Range, F., Huber, L., Miklósi, Á., Virányi, Zs. 2012. Does the A-not-B error in adult pet dogs indicate sensitivity to human communication? Animal Cognition, 15: 737-743.
Physical cognition
- Pongrácz, P., Miklósi, Á., Dóka, A., Csányi, V. 2003. Successful application of video-projected human images for signalling to dogs. Ethology, 109: 809-821.
- Péter, A., Miklósi, Á., Pongrácz, P. 2013. Domestic dogs’ (Canis familiaris) understanding of projected video images of a human demonstrator in an object-choice task. Ethology, 119: 898–906.
Memory
- Pongrácz, P., Benedek, V., Enz, S., Miklósi, Á. 2012. The owners’ assessment of „everyday dog memory” A questionnaire study. Interaction Studies, 13: 386-407.
- Fugazza, C., Miklósi, Á. 2014. Deferred imitation and declarative memory in domestic dogs. Animal Cognition, 17: 237-247.
Personality
- Kubinyi, E., Turcsán, B., Miklósi, Á. 2009. Dog and owner demographic characteristics and dog personality trait associations. Behavioural Processes, 81: 392-401.
- Turcsán, B., Kubinyi, E., Mikósi, Á. 2011. Trainability and boldness traits differ between dog breed clusters based on conventional breed categories and genetic relatedness. Applied Animal Behaviour Science, 132: 61-70.
- Turcsán, B., Range, F., Virányi, Zs., Miklósi, Á., Kubinyi, E. 2012. Birds of a feather flock together? Perceived personality matching in owner–dog dyads. Applied Animal Behaviour Science, 140: 154-160.
- Kis, A., Turcsán, B., Miklósi, Á., Gácsi, M. 2012. The effect of the owner’s personality on the behaviour of owner-dog dyads. Interaction Studies, 13: 371-383.
- Mirkó, E., Kubinyi, E., Gácsi, M., Miklósi, Á. 2012. Preliminary analysis of an adjective-based dog personality questionnaire developed to measure some aspects of personality in the domestic dog (Canis familiaris). Applied Animal Behaviour Science, 138: 88– 98.
- Mirkó, E., Dóka, A., Miklósi, Á. 2013. Association between subjective rating and behaviour coding and the role of experience in making video assessments on the personality of the domestic dog (Canis familiaris). Applied Animal Behaviour Science, 149: 45-54.
- Temesi, A., Turcsán B., Miklósi, Á. 2014. Measuring fear in dogs by questionnaires: An exploratory study toward a standardised inventory. Applied Animal Behaviour Science, 161: 121-130.
- Ákos, Zs., Beck, R., Nagy, M., Vicsek, T., Kubinyi, E. 2014. Leadership and path characteristics during walks are linked to dominance order and individual traits in dogs. PLoS Comput Biol, 10(1): e1003446
- Kubinyi, E., Gosling, S., Miklósi, Á. 2015. A comparison of rating and coding behavioural traits in dogs. Acta Biologica Hungarica. 66:27-40.
Behaviour genetics
- Héjjas, K., Vas, J., Topál, J., Szántai, E., Rónai, Zs., Székely, A., Kubinyi, E., Horváth, Zs., Sasvári-Székely, M., Miklósi, Á. 2007. Association of polymorphisms in the dopamine D4 receptor gene and the activity-impulsivity endophenotype in dogs. Animal Genetics, 38: 629–633.
- Héjjas, K., Vas, J., Kubinyi, E., Sasvári-Székely, M., Miklósi, Á., Rónai, Z. 2007. Novel repeat polymorphisms of the dopaminergic neurotransmitter genes among dogs and wolves.Mammalian Genome, 18: 871-879.
- Héjjas, K., Kubinyi, E., Rónai, Zs., Székely, A., Vas, J., Miklósi, Á., Sasvári-Székely, M., Kereszturi, E. 2009. Molecular and behavioral analysis of the intron 2 repeat polymorphism in canine dopamine D4 receptor gene. Genes, Brain, Behaviour, 8: 330-336.
- Kubinyi, E., Vas, J., Héjjas, K., Ronai, Zs., Brúder, I., Turcsán, B., Sasvári-Székely, M., Miklósi, Á. 2012. Polymorphism in the tyrosine hydroxylase (TH) gene is associated with activity-impulsivity in German Shepherd dogs. PLoS One, 7(1): e30271.
- Wan, M., Héjjas, K., Rónai, Zs., Elek, Zs., Sasvári-Székely, M., Champagne, F.A., Miklósi, Á., Kubinyi, E. 2013. DRD4 and TH gene polymorphisms are associated with activity, impulsivity and inattention in Siberian Husky dogs. Animal Genetics, 44: 717-727.
- Kis, A., Bence, M., Lakatos, G., Pergel, E., Turcsán, B., Pluijmakers, J., Vas, J., Elek, Zs., Brúder, I., Földi, L., Sasvári-Székely, M., Miklósi, Á., Rónai, Zs., Kubinyi, E. 2014. Oxytocin receptor gene polymorphisms are associated with human directed social behavior in dogs (Canis familiaris).
Vocalizations
- Pongrácz, P., Miklósi, Á., Molnár, Cs., Csányi, V. 2005. Human listeners are able to classify dog barks recorded in different situations. Journal of Comparative Psychology, 119: 136-144.
- Molnár, Cs., Pongrácz, P., Dóka, A., Miklósi, Á. 2006. Can humans discriminate between dogs on the base of the acoustic parameters of barks? Behavioural Processes, 73: 76-83.
- Pongrácz, P., Molnár, Cs., Miklósi, Á. 2006. Acoustic parameters of dog barks carry emotional information for humans. Applied Animal Behaviour Science, 100: 228-240.
- Maros, K., Pongrácz, P., Bárdos, Gy., Molnár, Cs., Faragó, T., Miklósi, Á. 2008. Dogs can discriminate barks from different situations. Applied Animal Behaviour Science, 114: 159–167.
- Molnár, Cs., Kaplan, F., Roy, P., Pachet, F., Pongrácz, P., Dóka, A., Miklósi, Á. 2008. Classification of dog barks: a machine learning approach. Animal Cognition, 11:389–400.
- Molnár, Cs., Pongrácz, P., Faragó, T., Dóka, A., Miklósi, Á. 2009. Dogs discriminate between barks: The effect of context and identity of the caller. Behavioural Processes, 82: 198-201.
- Molnár, Cs., Pongrácz, P., Miklósi, Á. 2010. Seeing with ears: Sightless humans’ perception of dog bark provides a test for structural rules in vocal communication. The Quarterly Journal of Experimental Psychology, 63: 1004-1013.
- Pongrácz, P., Molnár, Cs., Miklósi, Á. 2010. Barking in family dogs: An ethological approach. The Veterinary Journal, 183: 141-147.
- Pongrácz, P., Molnár, Cs., Dóka, A., Miklósi, Á. 2011. Do children understand man’s best friend? Classification of dog barks by pre-adolescents and adults. Applied Animal Behaviour Science, 135: 95-102.
- Bálint, A., Faragó, T., Dóka, A., Miklósi, Á., Pongrácz, P. 2013. ‘Beware, I am big and non-dangerous!’–Playfully growling dogs are perceived larger than their actual size by their canine audience. Applied Animal Behaviour Science, 148: 128-137.
- Faragó, T., Andics, A., Devecseri, V., Kis, A., Gácsi, M., Miklósi, Á. 2014. Humans rely on the same rules to assess emotional valence and intensity in conspecific and dog vocalizations. Biology Letters, 10: 20130926.
- Larranaga, A., Bielza, C., Pongrácz, P., Faragó, T., Bálint, A., Larranaga, P. 2015. Comparing supervised learning methods for classifying sex, age, context and individual Mudi dogs from barking. Animal Cognition, 18: 405-421.
- Andics, A., Gácsi, M., Faragó, T., Kis, A., Miklósi, Á. 2014. Voice-sensitive regions in the dog and human brain are revealed by comparative fMRI. Current Biology, 24: 574-578.
Emotions
- Sümegi, Zs., Oláh, K., Topál, J. 2014. Emotional contagion in dogs as measured by change incognitive task performance. Applied Animal Behaviour Science, 160: 106-115.
- Sümegi, Zs., Gácsi, M., Topál, J. 2014. Conditioned placebo effect in dogs decreases separation related behaviours. Applied Animal Behaviour Science, 159: 90-98.
- Turcsán, B., Szánthó, F., Miklósi, Á., Kubinyi, E. 2015. Fetching what the owner prefers? Dogs recognize disgust and happiness in human behaviour. Animal Cognition, 18: 83-94.
Cognitive neuroscience
- Andics, A., Gácsi, M., Faragó, T., Kis, A., Miklósi, Á. 2014. Voice-sensitive regions in the dog and human brain are revealed by comparative fMRI. Current Biology, 24: 574-578.
- Kis, A., Szakadát, S., Kovács, E., Gácsi, M., Simor, P., Gombos, F., Topál, J., Miklósi, Á., Bódizs, R. 2014. Development of a non-invasive polysomnography technique for dogs (Canis familiaris).Physiology & Behavior, 130: 149–156.
Ethorobotics
- Kaplan, F., Oudeyer, P.-Y., Kubinyi, E., Miklósi, Á. 2002. Robotic clicker training. Robotics and Autonomous Systems, 38: 197-206.
- Kubinyi, E., Miklósi, Á., Kaplan, F., Gácsi, M., Topál, J., Csányi, V. 2004. Social behaviour of dogs encountering AIBO, an animal-like robot in a neutral and in a feeding situation. Behavioural Processes, 65: 231-239.
- Kubinyi, E., Pongrácz, P., Miklósi, Á. 2010. Can you kill a robot nanny? Ethological approach to the effect of robot caregivers on child development and human evolution (comment). Interaction Studies, 11: 214-219.
- Gergely, A., Petró, E., Topál, J., Miklósi, Á. 2013. What are you or who are you? The emergence of social interaction between dog and an Unidentified Moving Object (UMO). PLoS ONE, 8: e72727.
- Miklósi, Á., Gácsi, M. 2012. On the utilization of social animals as a model for social robotics.Frontiers in Psychology, 3: 75.
- Gácsi, M., Szakadát, S., Miklósi, Á. 2013. Assistance dogs provide a useful behavioral model to enrich communicative skills of assistance robots. Frontiers in Psychology, 4: 971.
- Faragó, T., Gácsi, M., Korcsok, B., Miklósi, Á. 2014. Why is a dog-behaviour-inspired social robot not a doggy-robot? Interaction Studies, 15: 224-232.
- Faragó, T., Miklósi, Á., Korcsok, B., Száraz, J., Gácsi, M. 2014. Social behaviours in dog-owner interactions can serve as a model for designing social robots. Interaction Studies, 15: 143-172.
- Lakatos, G., Janiak, M., Malek, L., Muszynski, R., Konok, V., Tchon, K., Miklósi, Á. 2014. Sensing sociality in dogs: What may make an interactive robot social? Animal Cognition, 17: 387-397.
Automated behaviour coding
- Gerencsér, L., Vásárhelyi, G., Nagy, M., Vicsek, T., Miklósi, Á. 2013. Identification of behaviour in freely moving dogs (Canis familiaris) using inertial sensors. PLoS ONE, 8(10): e77814.
Cognitive bias
- Kis, A., Hernádi, A., Kanizsár, O., Gácsi, M., Topál, J. 2015. Oxytocin induces positive expectations about ambivalent stimuli (cognitive bias) in dogs. Hormones and Behavior, 69:1-7.
Other domestic animals
- Miklósi, Á., Pongrácz, P., Lakatos, G., Topál, J., Csányi, V. 2005. A comparative study of the use of visual communicative signals in interactions between dogs (Canis familiaris) and humans and cats (Felis catus) and humans. Journal of Comparative Psychology, 119: 179-186.
- Maros, K., Boross, B., Kubinyi, E. 2010. Approach and follow behaviour – possible indicators of the human-horse relationship. Interaction Studies, 11: 410-427.
- Maros, K., Gácsi, M., Miklósi, Á. 2008. Comprehension of human pointing gestures in horses (Equus caballus). Animal Cognition, 11: 457-66.
- Hernádi, A., Kis, A., Turcsán, B., Topál, J. 2012. Man’s underground best friend: Domestic ferrets, unlike the wild forms, show evidence of dog-like social-cognitive skills. PLoS One, 7(8): e43267.
For more papers see Family Dog Project’s publications.